Thermoelectric energy converters offer the possibility of direct conversion of thermal energy into electrical energy due to the coupling of molecular transport mechanisms. Compared to semiconductor-based thermogenerators, which are mainly used for large temperature differences, electrochemical thermocells based on a polymer electrolyte membrane represent a promising possibility for the conversion of low-temperature heat. Their design is similar to that of a PEM fuel cell, but in contrast to the PEM FC, a hydrogen/water vapor mixture of different temperatures flows around the two electrodes of the thermocell. The imposed temperature gradient leads to an electrical potential difference between the anode and the cathode, since the chemical potential of the half cells changes depending on the temperature. Thus, in a thermocell, the temperature gradient, the gradient in chemical potential, and the electrical potential gradient are closely coupled. These coupling processes can be characterized using the thermodynamics of irreversible processes. It is assumed that the fluxes occurring in the cell represent a linear combination of the totality of the driving forces. This linear relationship is described by means of so-called phenomenological coefficients. In contrast to purely empirical approaches such as Fick's, Fourier's or Ohm's law, the phenomenological coefficients take into account all interactions and superpositions of the driving forces and fluxes occurring in the cell.
At the Institute of Thermodynamics, both theoretical and experimental investigations of the PEM thermocell are carried out. In addition to the development of detailed models for the physical description of the transport mechanisms in the thermocell, measurements are carried out at the institute for the precise determination of the phenomenological coefficients required for the reliable description of the coupling processes in the thermocell.



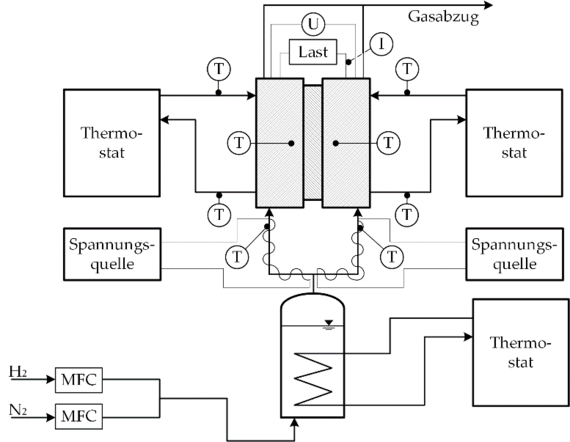
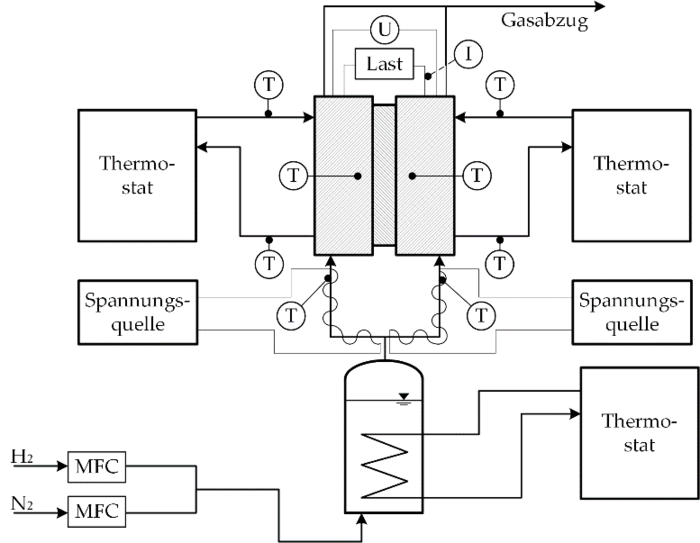

Processing






